Setting and Data
Setting
Data were taken from the BRISK-FL study- a randomised phase III study to compare the overall survival of brivanib versus sorafenib in patients with advanced HCC (aHCC). Patients were recruited from across Asia, Europe, America, Australia and Africa.
Estimand
Patients
Patients were eligible if they had not received any prior systemic therapy for aHCC and had a Child-Pugh A liver function score. Patients had to have an Eastern Cooperative Oncology Group (ECOG) Score of 0 or 1 and at least one untreated measurable lesion by computed tomography or magnetic resonance imaging. Patients with any prior use of systemic anticancer chemotherapy, immunotherapy or targeted agents for HCC (except for sorafenib) were excluded.
Intervention
Patients received brivanib as 800mg orally every day.
Outcome
The primary outcome is overall survival (OS) measured as the time from randomisation until death by any cause.
Data
The dataset consisted of 454 patients of whom 204 (45%) observed an event and 250 (55%) did not. The median overall survival (95% CI) was 16.2 (14.3, 17.6) months.
Model Covariates
The covariates selected for inclusion in the model were
Eastern Cooperative Oncology Group (ECOG) Score, Extra-hepatic spread (EHS),
bilirubin (BIL), tumour size, age, albumin (ALB) and alpha-fetoprotein (AFP)
| N = 454 | |
|---|---|
| Eastern Cooperative Oncology Group Score | |
| 0 | 199 (44%) |
| 1 | 230 (51%) |
| 2 | 25 (5.5%) |
| Extrahepatic Spread | |
| Positive | 164 (36%) |
| Negative | 290 (64%) |
| Bilirubin | 1.13 (0.95, 1.34) |
| Tumour Size | 7.35 (5.57, 9.64) |
| Age | 68 (62,74) |
| Albumin | 3.80 (3.40,4.20) |
| AFP | 2.70 (1.79,3.76) |
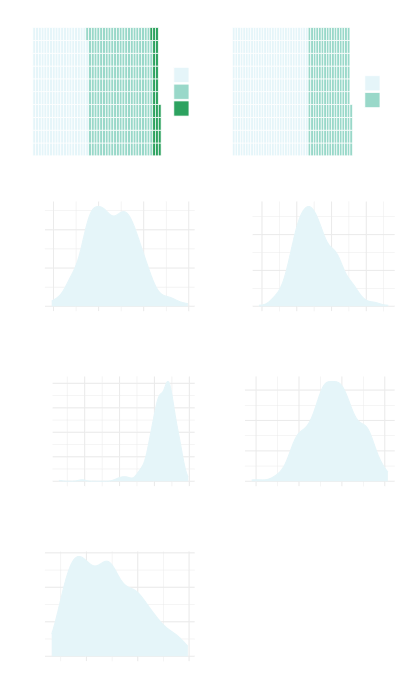
Model
The model constructed was a flexible parametric survival model using a spline function to model the underlying cumulative hazard function. Four internal knots were chosen and were placed at the timepoints 3, 6 12, and 24 months.
Model Construction
Include details here about the process of fitting the model. E.G. backwards stepwise procedure based on model AIC.
Model Fit
Some text to describe what is provided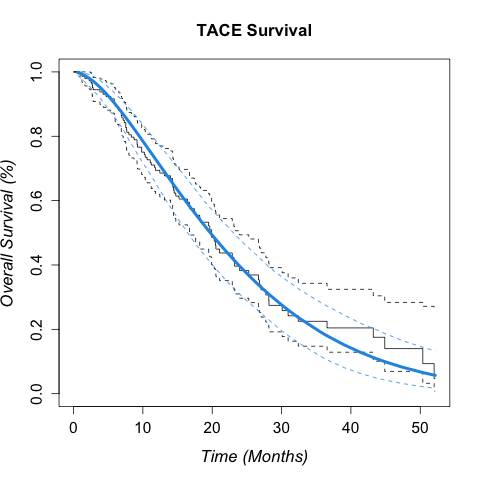
Model Estimates
| Estimate (se) | HR (95% CI) | |
|---|---|---|
| γ0 | -5.65 (1.02) | 0.00 (0 - 0.026) |
| γ1 | 1.98 (0.47) | 7.27 (2.906 - 18.173) |
| γ2 | 0.06 (0.25) | 1.07 (0.655 - 1.738) |
| γ3 | 0.42 (0.5) | 1.52 (0.57 - 4.049) |
| γ4 | -1.62 (0.67) | 0.2 (0.053 - 0.728) |
| γ5 | 3.58 (1.18) | 36.04 (3.578 - 363.04) |
| ECOG1 | 0.28 (0.15) | 1.32 (0.981 - 1.78) |
| ECOG2 | 0.59 (0.31) | 1.81 (0.981 - 3.333) |
| Extrahepatic Spread: Positive Vs Negative | 0.52 (0.15) | 1.68 (1.248 - 2.251) |
| Bilirubin | 1.41 (0.29) | 4.09 4.09 (2.326 - 7.208) |
| Tumour size | 0.08 (0.03) | 1.08 (1.03 - 1.141) |
| Age | 0.02 (0.01) | 1.02 (1.002 - 1.03) |
| Albumin | -0.61 (0.14) | 0.54 (0.408 - 0.72) |
| Alpha-fetoprotein | 0.07 (0.06) | 1.08 ((0.959 - 1.209)) |
Model Prediction
See how this model can be used to predict survival!Validation
Details on the validation of the model:
Validation Details
Validation are reported in term of Calibration, Discrimination and Somers' D.
Calibration is reported in terms of the Mallows C-Statistic and by regressing
the fitted linear predictor against the outcome (Slope). The linear predictor is
derived using the model's coefficients.
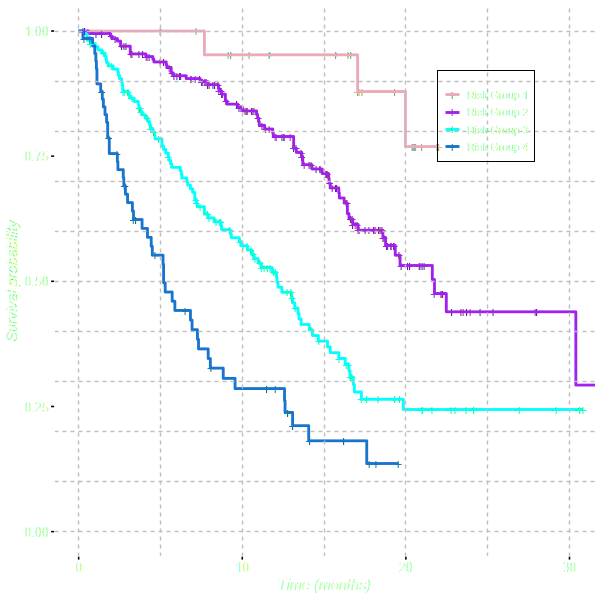
Discrimination is evaluated by categorising patients into 4 risk groups.
Risk groups are generated by using the 5th, 50th and 85th centiles of the
linear predictor. The risk groups are compared graphically
and the relative risk is evaluated by fitting a
univariable Cox Proportional Hazards Model.
Calibration
| est (se) | |
|---|---|
| C-Statistic | 0.70 (0.017) |
| Slope | 1.00 (0.101) |
| Somers' D | 0.41 |
Discrimination
| est (se) | HR (95% CI) | |
|---|---|---|
| Risk Group 1 | ||
| Risk Group 2 | 0.06 (0.26) | 1.06 (0.63, 1.77) |
| Risk Group 3 | 1.06 (0.24) | 2.89 (1.81, 4.61) |
| Risk Group 4 | 1.82 (0.26) | 6.20 (3.70, 10.37) |
Use this model
Download this model and learn how to use it by visiting